What Element Has 35 Protons 44 Neutrons and 36 Electrons
Matter is made up of tiny building blocks called Atoms. There are multiple ways of grouping the elements but they are commonly divided into metals semimetals metalloids and nonmetals.
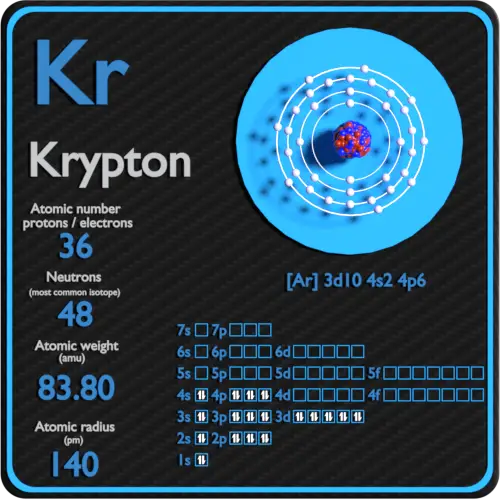
Krypton Protons Neutrons Electrons Electron Configuration
Thermal electrons are released from a heated element.
. The atoms of an element that have different numbers of neutrons are called isotopes of that element. Number of Neutrons - How to find the Number of Neutrons We can identify the number of neutrons in an atom or. The neutron has zero charge and its mass is comparable with the mass of a positively charged proton.
Everything that takes up space called Matter has weight volume and mass. The table of nuclides comprises all the known nuclides. Trivial name of Sodium is alkali metals.
An ion is a charged atom with more or fewer electrons than protons. The most common nuclide of the common chemical element lead 208 Pb has 82 protons and 126 neutrons for example. Sodium is a alkali metal element.
The atomic weight values may be different from one periodic table to another because its a calculated number based on the weighted average of. What is an atom. The exact size of the atom.
It is denoted by. The purest type of atom is called an element. You can easily estimate the atomic weight.
Normally an atom has equal numbers of protons and electrons. Trivial name of Lead is tetrels crystallogens. Even though it is not a chemical element the neutron is included in this table.
The number of protons the number of neutrons and the number of electrons an atom has determines what the element it is. Element 82 of Periodic table is Lead with atomic number 82 atomic weight 2072. An element is a.
It is usually 2 to 25 times the atomic number. Element 11 of Periodic table is Sodium with atomic number 11 atomic weight 2298977. Its the sum of the mass of protons and neutrons in an atom electrons contribute negligible mass but you may notice it isnt the value youd get if you assumed the atom had an equal number of protons and neutrons.
Since each proton and neutron has mass equal to 4 u therefore mass of an atom must be the same as the sum of protons and neutrons present in the nucleus. And electrons are negative. As the electrons have negligible mass the entire mass of the atom is that of protons and neutrons present in its nucleus.
The number of nucleons both protons and neutrons in the nucleus is the atoms mass number and each isotope of a given element has a different mass number. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. Know everything about Lead Facts Physical Properties Chemical Properties Electronic configuration.
Youll find more specific groups like transition metals rare earths alkali metals alkaline earth halogens and noble gasses. The free neutron has a mass of 939 565 4133 eVc 2 or 1674 927 471 10 27 kg or 1008 664 915 88 Da. Know everything about Sodium Facts Physical Properties Chemical Properties Electronic configuration Atomic.
To understand the association between the number of Protons and Neutrons and an element we need to ask a basic question. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. The latter is known as mass number of an element.
The atomic weight of an element is the average number of protons plus neutrons. All neutral atoms of the same element have the same number of protons and electrons but interestingly they often differ in the number of neutrons. Its monatomic form H is the most abundant chemical substance in the Universe constituting.
Both neutrons and protons can be seen as matter waves. Neutrons have been used in scattering experiments to determine crystalline structures of solids from interference patterns formed by neutron matter waves. Lead symbol Pb has a Face Centered Cubic structure and SlateGray color.
This means that atoms of the same element while having the same atomic number can have different mass numbers. Atoms are tiny at least 100 times smaller than the width of a human hair. Atoms are composed of three kinds of smaller particles called protons neutrons and electrons.
Sodium symbol Na has a Body Centered Cubic structure and Silver color. For example carbon-12 carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12 13 and 14 respectively. The atomic number of carbon is 6 which means that.
Lead is a post-transition metal element.

Comments
Post a Comment